
The longest-lived radioisotope is 81mKr with a half-life of 230,000 years. The radioactive decay of these short-lived radioisotopes causes trace amounts of naturally occurring radioactivity in krypton.

All but four of the remaining 19 natural isotopes are very short-lived radioisotopes that decay rapidly to stable isotopes of other elements: 82Kr decays to 82Se 89Kr decays to 89Sr 90Kr decays to 90Zr 91Kr decays to 91Zr 92Kr decays to 92Zr 94Kr decays to 94Ru 96Kr decays to 96Ru 98Kr decays to 98Ru 100Kr decays to 100Rh 101Kr decays to 101Rh 102Kr decays to 102Rh and 104Kr decays to 104Pd.
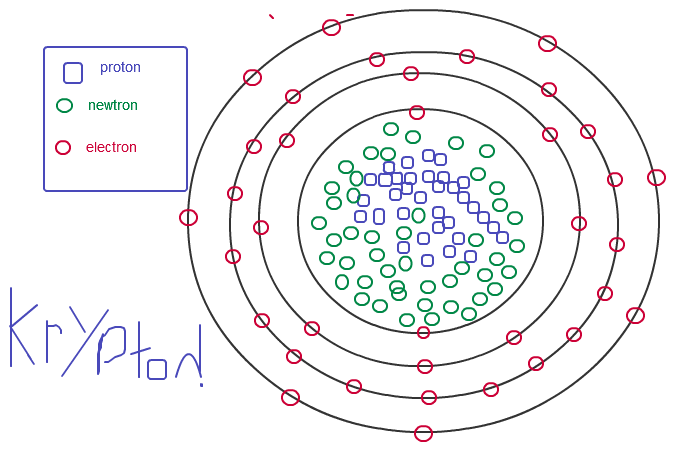
The most common isotope by far is 86Kr (75%), followed by 84Kr (25%). In nature, krypton is found isotopes with mass numbers from 84 to 103. Krypton may also be described as being inner transition metal because it has a partially filled d subshell. The configuration of the outermost electrons of krypton can be represented as Kr(Ne):4s2 3d10 4p6, indicating that its outermost shells contain eight electrons. Krypton’s low boiling point of −153.22 ☌ makes it useful for cooling infrared detectors. In addition, krypton does not absorb ultraviolet radiation, making it useful for fillings in high-powered bulbs and as a non-reactive coating for other light sources such as solar panels. With a relatively high atomic number, krypton is relatively rare: Earth’s atmosphere contains 0.0001% krypton by volume.Īs with all noble gases, krypton is chemically inert and forms no known compounds at room temperature. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. It is a member of group 18 (noble gases) elements. Krypton is a chemical element with the symbol Kr and atomic number 36.


 0 kommentar(er)
0 kommentar(er)
